A Rapid Evolving Landscape
In the rapidly evolving landscape of the pharmaceutical and biotech industries, Contract Development and Manufacturing Organisations (CDMOs) have emerged as pivotal players. These organisations, which offer comprehensive services from drug development through to manufacturing, are integral in bringing new therapies to market swiftly and efficiently. The rapid emergence of Advanced Therapeutic Manufacturing products (ATMP) provides significant opportunities and challenges for CDMOs. Due to the nature of Advanced Therapeutics a greater emphasis is required on streamlining of operational process, development of repeatable and replicable manufacturing processes, to drive down waste whilst maintaining quality, reducing costs and improving efficiency. Scaling ATMP’s operations present a unique set of challenges for CDMO and the path to success is fraught with challenges, including stringent regulatory requirements, high operational costs, and the need for innovation. This context underscores the thesis that operational excellence is not just beneficial but essential for CDMOs. Achieving and maintaining high operational standards is the linchpin for CDMOs to remain competitive, ensure product quality, comply with global regulations, and ultimately, sustain growth. Operational excellence, therefore, is not merely an operational goal but a strategic imperative for any CDMO aiming for industry leadership and long-term success.
Understanding Operational Excellence
Operational excellence within the framework of CDMOs is a multifaceted concept that extends beyond mere efficiency. It encapsulates a commitment to continuously improve processes, ensuring the highest quality of products, compliance with stringent regulatory standards, and fostering innovation. At its core, operational excellence involves streamlining manufacturing processes, minimising waste, and optimising resource use, thereby enhancing overall productivity. It also entails a robust quality management system that guarantees products meet the exacting standards of regulatory bodies and clients. Furthermore, operational excellence drives a culture of continuous improvement and innovation, enabling CDMOs to adapt to the ever-changing demands of the pharmaceutical and biotech sectors. This comprehensive approach ensures that CDMOs can deliver on time, within budget, and to the highest quality standards, making operational excellence a cornerstone of their strategic advantage.
Example Benefit #1: Cleaning Operations
- Materials Saving: £130,000
- Time saving: 1614 hours = £8,322
- Total Savings: £138,322
The Competitive Edge
In the highly competitive landscape of Advanced Therapeutics operational development, operational excellence serves as a critical differentiator for CDMOs. It is the engine that powers efficiency, quality, and innovation, attributes that are paramount in attracting and retaining clients. CDMOs that excel operationally often showcase reduced production timelines, enhanced capacity for successfully implementing complex projects, and a track record in digital and operational innovation. These capabilities not only position them as leaders but also as reliable partners in the eyes of their existing and potential clients. For instance, a CDMO that has mastered operational excellence can navigate the complexities of producing novel biologics, handle high volume productivity, and pivot swiftly to emerging therapeutic areas. Such agility and reliability are invaluable in a sector where speed to market can significantly impact the commercial success of a new treatment. Operational excellence, therefore, is not just a matter of internal efficiency but a strategic asset that elevates a CDMO’s market position.
Example Benefit #2:
- Reduced Turnaround time from 19 days – <3 days
- Failure Rates reduced from 28% to <5%
ATMP Market Opportunity
The ATMP market is competitive, with multiple players vying for attention and market share. Organisations that achieve Operational Excellence gain a competitive advantage by delivering products faster, at a higher quality, and often at a more competitive price point.
The image below highlights just some of the drivers and benefits Operational Excellence offers ATMPs.
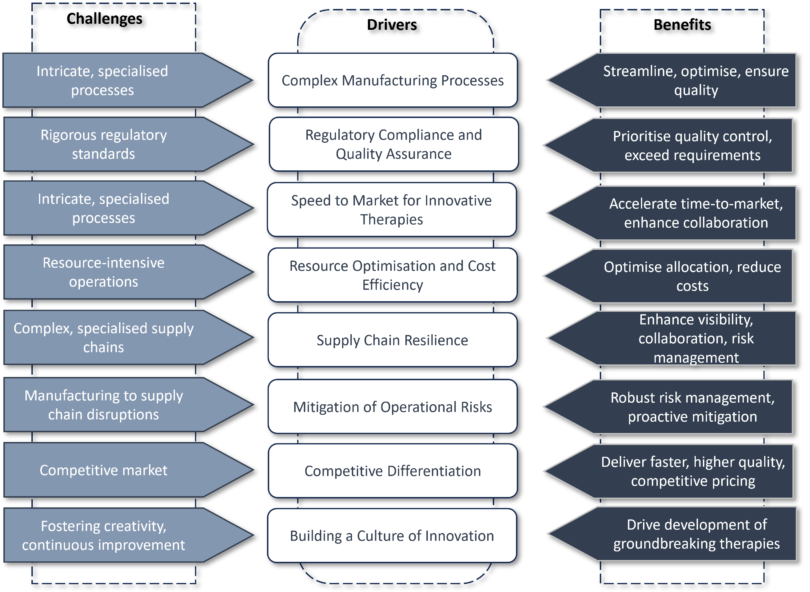
Quality and Compliance
Quality and compliance are non-negotiable in the life science industry. Operational excellence ensures that CDMOs not only meet but exceed the rigorous standards set by regulatory authorities. A commitment to operational excellence manifests in robust quality control systems, meticulous documentation, and a culture that prioritises patient safety above all. This unwavering focus on quality and compliance mitigates the risk of regulatory infractions, which can lead to costly delays, fines, or even the rejection of drug applications. Moreover, operational excellence in quality and compliance builds trust with clients, who can be assured that their projects are managed according to the highest standards. For CDMOs, this trust translates into long-term partnerships and a strong reputation in the industry, further underlining the indispensable role of operational excellence.
Example Benefit #3: Waste optimisation
- Materials Saving: £3,959
- Time saving: 122 hours = £3,959
Efficiency and Cost-effectiveness
Operational excellence directly impacts a CDMO’s ability to operate efficiently and manage costs effectively. By optimising processes and eliminating waste, CDMOs can achieve significant savings, which can then be passed on to clients or reinvested in innovation and capacity expansion. This efficiency not only enhances profitability but also makes CDMOs more competitive in terms of pricing and project turnaround times. In a market where cost pressures are constantly mounting, the ability to offer high-quality services at competitive prices is a distinct advantage. Furthermore, operational excellence enables CDMOs to scale their operations seamlessly, accommodating growth without compromising on quality or efficiency. This scalability is crucial for meeting the dynamic needs of clients and responding to fluctuations in demand, making operational excellence a key driver of financial health and business sustainability.
Example Benefit #4: Recruitment
- Saving: £30,000
- Time saving: 1125 hours.
Innovation and Scalability
Operational excellence is intrinsically linked to a CDMO’s capacity for innovation and scalability. A streamlined and efficient operation creates the foundation for investing in new technologies, research and development, and expanding production capabilities. This investment in innovation not only enhances a CDMO’s service offering but also ensures that it can adapt to future challenges and opportunities in the pharmaceutical and biotech sectors. Scalability, facilitated by operational excellence, allows CDMOs to quickly adjust to increasing demands, expand into new therapeutic areas, and undertake complex manufacturing projects. This agility is a competitive advantage in an industry characterised by rapid advancements and evolving market needs. Thus, operational excellence is not just about maintaining the status quo but driving growth and ensuring a CDMO’s relevance and leadership in the future.
Example Benefit #5: Scaling Throughput
- Increase in sample processing from 3,000 to 60,000 samples a week in three weeks.
Challenges to Achieving Operational Excellence
Despite its undeniable benefits, achieving operational excellence is fraught with challenges. CDMOs often face obstacles such as legacy systems, cultural resistance to change, and the complexity of managing complex supply chains. Overcoming these challenges requires a strategic approach that includes investing in technology, focusing on the human dimension of change, fostering a culture of continuous improvement, and developing robust supply chain management practices. Leadership commitment is also crucial in driving the organisational changes necessary for operational excellence. By addressing these challenges head-on, CDMOs can lay the foundation for operational excellence, ensuring they can meet the current and future demands of the life science and biotech industries.
Conclusion
Operational excellence is not just an operational goal but a strategic imperative for CDMOs. It is the foundation upon which quality, efficiency, compliance, and innovation are built. In the competitive and regulated environment of pharmaceutical manufacturing, operational excellence distinguishes leaders from followers. It enables CDMOs to deliver superior quality products, comply with global regulatory standards, manage costs effectively, and innovate for future growth. Despite the challenges, the pursuit of operational excellence is an essential journey worth embarking on for any CDMO committed to long-term success and industry leadership. As the pharmaceutical and biotech sectors continue to evolve, operational excellence will remain a critical factor in determining which CDMOs thrive, and which fall by the wayside.
Biomaitcs delivers Operational Excellence only for the Life Science sector. To find out how Operational Excellence can make a real difference to your operations and service offer, contact us and talk to our Director of Business Excellence Service.



